Unveiling the Enigma: Are Enantiomers Mirror Images? A Deep Dive into Molecular Chirality and Visual Symmetry
At Tophinhanhdep.com, we are passionate about the power of visuals – from stunning wallpapers and aesthetic backgrounds to intricate digital art and high-resolution photography. We explore the world through lenses of beauty, design, and precision. But beyond the macroscopic canvases we typically admire, lies a microscopic realm where visual symmetry, or the lack thereof, dictates fundamental properties. This realm is organic chemistry, and within it, one of the most fascinating concepts revolves around molecules that are “mirror images” of each other. Much like how a photographer captures the nuances of light and shadow, chemists observe and define subtle spatial arrangements that profoundly impact a molecule’s behavior. The question, “are enantiomers mirror images?”, delves into the heart of this molecular aesthetics, revealing a world of structural elegance and functional distinction.
Imagine holding up your left hand to a mirror; what you see is a reflection that perfectly matches your right hand. Your two hands are mirror images. Yet, try to superimpose them, palm to palm, thumb to thumb. You’ll find they don’t perfectly overlap – they are non-superimposable. This seemingly simple observation introduces us to the profound concept of chirality, or “handedness,” a property not just of hands but of countless objects, from spiral staircases to certain seashells. In the intricate world of molecules, this same principle applies, giving rise to fascinating pairs known as enantiomers. These are molecules that possess identical chemical formulas and atom connectivity, yet their spatial arrangement—their 3D “visual design”—is distinctly different, making them non-superimposable mirror images. Understanding this concept is not just an academic exercise; it’s crucial for fields ranging from pharmaceutical development to material science, highlighting how subtle visual distinctions at the molecular level can lead to vastly different outcomes.
The Essence of Chirality: Understanding Non-Superimposable Mirror Images
The journey into enantiomers begins with grasping the fundamental concept of non-superimposable mirror images. This idea, so clearly demonstrated by our own left and right hands, is central to molecular chirality.
What Defines an Enantiomer?
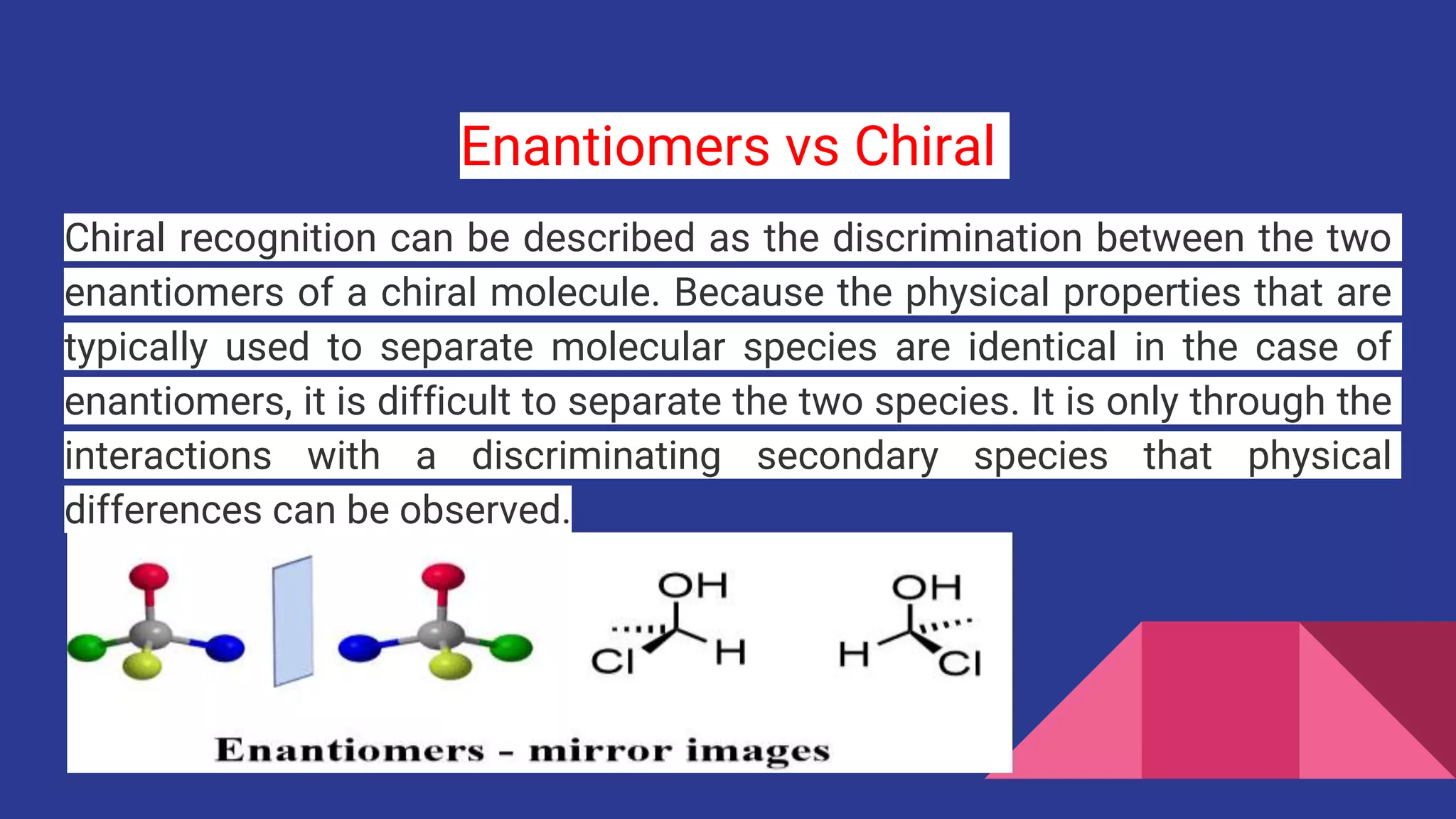
Enantiomers are precisely defined as stereoisomers that are non-superimposable mirror images of one another. The term “isomer” means they have the same molecular formula. “Stereoisomer” further specifies that their atoms are connected in the same sequence, but their three-dimensional arrangement in space differs. It is this spatial difference, analogous to how an artist might arrange elements in a visual composition, that gives enantiomers their unique character.
To elaborate on the “non-superimposable” aspect, let’s revisit the hand analogy. Your left hand is a mirror image of your right. If you were to place your right hand flat on a table, and then try to place your left hand directly on top of it, in the exact same orientation (thumb on thumb, palm on palm), you would find it impossible without bending your fingers or breaking bonds in a molecular context. The two hands, despite being mirror images, cannot occupy the same space in the same orientation simultaneously.
Similarly, enantiomers possess the same chemical formula, the same types and number of atoms, and these atoms are connected in the same order. However, their distinct spatial arrangement prevents one from being perfectly aligned with its mirror image through simple rotations in space. This is a critical distinction, as molecules that are superimposable on their mirror images are termed “achiral” – lacking handedness. For instance, dichloromethane (CH2Cl2) is an achiral molecule. Its mirror image can be rotated to perfectly overlap with the original, much like a symmetrical vase can be perfectly superimposed on its mirror image. But if we consider a molecule like Bromochlorofluoromethane (CHBrClF), we enter the realm of chirality. Its mirror image cannot be simply rotated to match the original structure; bonds would have to be broken and reformed, demonstrating their non-superimposable nature. This is a testament to the elegant yet precise “visual design” that defines molecular structure, much like the precision required in high-resolution photography.
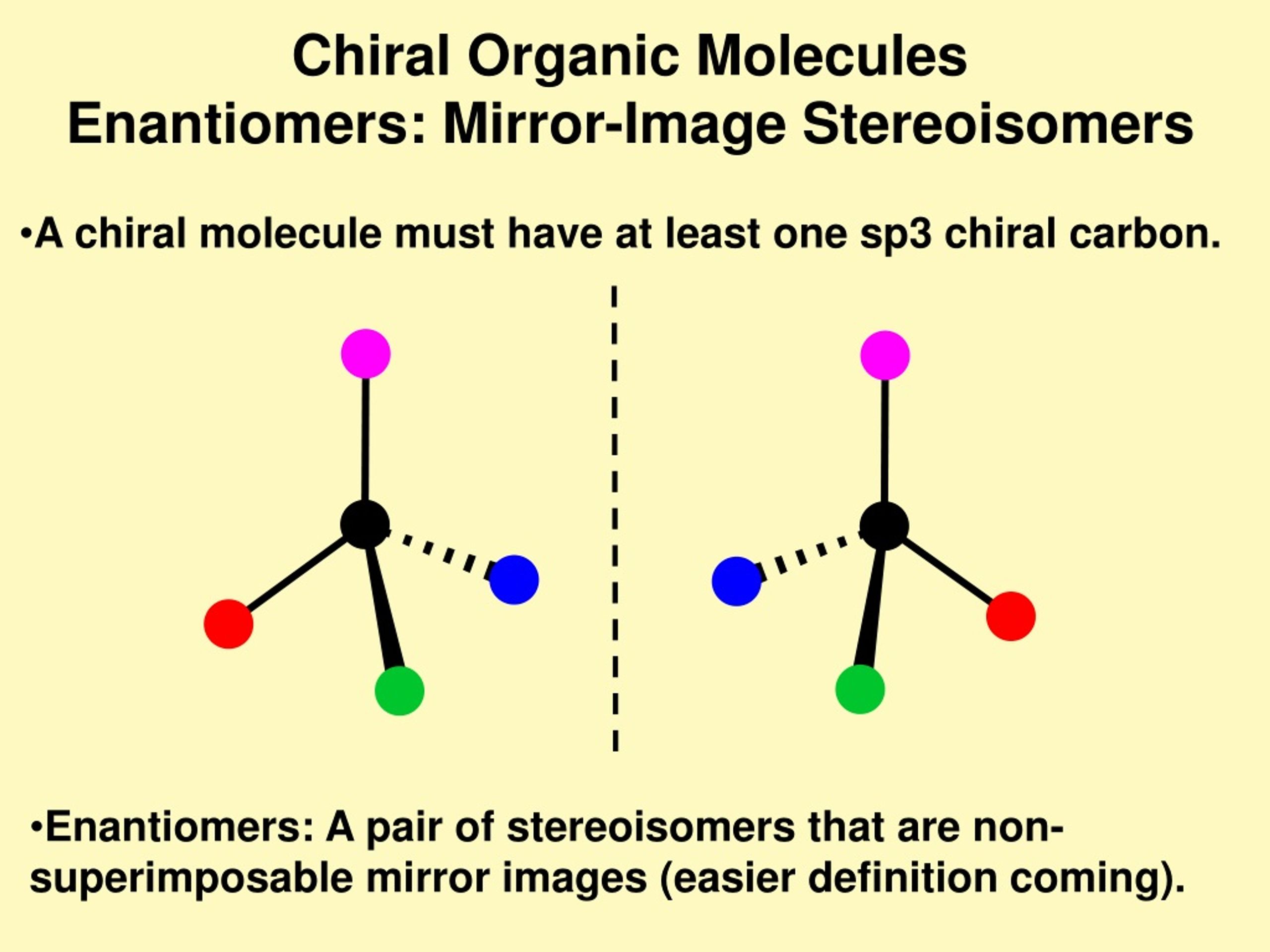
The Stereogenic Center: The Heart of Molecular Handedness
What gives a molecule this fascinating property of chirality? Most often, the origin lies in a specific point within its structure known as a stereogenic center, also frequently called a chiral center. For organic molecules, this usually refers to a carbon atom bonded to four different types of atoms or groups of atoms. This tetrahedral arrangement with four distinct substituents is the key to creating a non-superimposable mirror image. If even two of the substituents were identical, the molecule would typically possess a plane of symmetry, rendering it achiral and superimposable on its mirror image. This is akin to understanding the focal point in an abstract wallpaper design; that single point dictates the overall “handedness” or symmetry of the entire composition.
While carbon is the most common element to serve as a chiral center, it’s not the only one. Other atoms, such as nitrogen and phosphorus, can also exhibit chirality under certain conditions, adding layers of complexity to molecular architecture.
Chirality at Nitrogen: Nitrogen atoms, when part of a single-bonded system (like in amines), typically adopt a pyramidal shape, with a lone pair of electrons occupying one corner of a theoretical tetrahedron. If this nitrogen is bonded to three different groups, its configuration is technically chiral. However, a fascinating phenomenon known as “amine inversion” often prevents these nitrogen centers from being configurationally stable. At room temperature, the nitrogen atom rapidly inverts its configuration, passing through a planar transition state. This rapid interconversion between its mirror-image forms (R and S configurations) means that, unless special conditions are met (e.g., very low temperatures or constrained structures), chiral nitrogen centers usually don’t result in isolable enantiomers. It’s like trying to capture a perfect still image of a rapidly shifting, dynamic abstract art piece.
Chirality at Phosphorus: In contrast to nitrogen, phosphorus atoms can be much more stable chiral centers. Due to their larger size and different electronic properties, phosphorus compounds (like phosphate esters) with four different groups attached to the phosphorus atom can form stable, isolable enantiomers. This stability makes phosphorus chiral centers significant in biological systems and synthetic chemistry, demonstrating how the very slight differences in atomic properties can lead to profound structural and functional implications, much like the choice of a specific editing style can dramatically alter the perception of a beautiful photograph.
Understanding these stereogenic centers is paramount. Just as a graphic designer meticulously places elements to create a balanced visual, nature carefully arranges atoms around these centers, giving molecules their unique spatial identities and, consequently, their specific biological and chemical functions.
Visualizing Molecular Differences: R and S Configurations & Optical Activity
Having established what defines an enantiomer and where chirality originates, the next step is to understand how we visually classify and functionally differentiate these molecular mirror images. This involves a precise labeling system and observing a unique physical property.
Decoding Molecular Handedness: R and S Configurations
To unambiguously describe the specific three-dimensional arrangement of groups around a chiral center, chemists use the Cahn-Ingold-Prelog (CIP) system, which assigns an absolute configuration of either ‘R’ (from rectus, Latin for right) or ‘S’ (from sinister, Latin for left) to each stereogenic center. This is akin to providing a unique digital tag or metadata for a complex image, ensuring its precise identification.
The process involves a set of rules:
- Assign Priorities: Each atom directly attached to the chiral center is assigned a priority (1-4) based on its atomic number. The atom with the highest atomic number gets the highest priority (1), and the one with the lowest atomic number gets the lowest priority (4). If there’s a tie, we move out to the next atoms along the chain until a difference is found.
- Orient the Molecule: The molecule is then conceptually oriented so that the lowest priority group (priority #4, often a hydrogen atom) is pointing away from the viewer (represented by a dashed line in wedge-and-dash projections).
- Trace the Path: Once oriented, trace a path from the highest priority group (1) to the second-highest (2), and then to the third-highest (3).
- If this path follows a clockwise direction, the configuration is R.
- If this path follows an anticlockwise (counter-clockwise) direction, the configuration is S.
This R/S nomenclature provides a standardized “visual design language” to communicate the exact spatial arrangement of atoms. Enantiomers, being non-superimposable mirror images, will always have opposite R/S configurations at every corresponding chiral center (e.g., if one enantiomer is (R)-2-chlorobutane, its mirror image will be (S)-2-chlorobutane). This systematic approach highlights the precision inherent in molecular descriptions, much like using specific color codes or grid systems in graphic design to ensure accuracy and consistency. The ability to precisely define these configurations is what allows scientists to differentiate between molecules that, at first glance, appear almost identical, yet behave very differently in chiral environments.
The Dance of Light: Dextrorotatory, Levorotatory, and Racemic Mixtures
Beyond their R/S designations, enantiomers exhibit another fascinating difference: their interaction with plane-polarized light. This property, known as optical activity, is a key distinguishing feature and provides a direct, observable characteristic for these mirror-image molecules.
When ordinary light passes through a polarizer, only light waves oscillating in a single plane are allowed to pass, creating plane-polarized light. When this plane-polarized light encounters a solution containing a chiral molecule (an enantiomer), the plane of oscillation of the light is rotated. This phenomenon occurs because the electrons in the chiral molecules interact differently with the oscillating electric field of the light depending on the light’s direction of oscillation relative to the molecule’s asymmetric structure.
- An enantiomer that rotates plane-polarized light in a clockwise direction is termed dextrorotatory (from Latin dexter meaning right), denoted with a (+) sign.
- An enantiomer that rotates plane-polarized light in a counter-clockwise direction is termed levorotatory (from Latin laevus meaning left), denoted with a (-) sign.
It is important to note that the R/S configuration does not directly correlate with dextrorotatory or levorotatory properties. An (R) enantiomer could be either (+) or (-), and its (S) counterpart would then have the opposite optical rotation. The direction and magnitude of rotation can only be determined experimentally using a polarimeter. This is a powerful demonstration of how seemingly identical structures, when viewed through a different “lens” (in this case, polarized light), reveal their distinct properties.
A fascinating outcome of this property is observed in racemic mixtures. A racemic mixture contains equal amounts of both enantiomers of a chiral compound. Since one enantiomer rotates plane-polarized light clockwise and the other rotates it counter-clockwise by an equal magnitude, their effects cancel each other out. Consequently, a racemic mixture is optically inactive – it does not rotate plane-polarized light. This concept is vital in fields like pharmacology, as often only one enantiomer of a drug is biologically active, and its presence in a racemic mixture can be diluted or complicated by the inactive counterpart.
The ability of enantiomers to rotate plane-polarized light is a beautiful example of how subtle spatial arrangements lead to tangible, measurable differences. It’s like observing how different lighting conditions or color filters can drastically alter the mood and visual impact of a photograph, even if the core subject remains the same. This inherent “visual sensitivity” of chiral molecules underscores their unique place in chemistry and biology.
Drawing and Distinguishing Enantiomers: A Designer’s Perspective on Molecular Representation
Just as visual designers utilize specific tools and techniques to represent complex ideas, chemists employ conventions to draw and differentiate enantiomers. Understanding these methods is crucial for accurately communicating molecular structures and their fascinating three-dimensional characteristics.
Techniques for Representing Enantiomers
When dealing with the three-dimensional nature of molecules, especially those with chiral centers, specific drawing conventions are used. The most common is the wedge-and-dash representation, where solid wedges denote bonds coming out towards the viewer, and dashed wedges indicate bonds going away from the viewer.
There are two primary ways to draw the enantiomer of a given molecule:
-
The Mirror Reflection Method: Conceptually place an imaginary mirror next to the molecule and draw its reflection. Every atom and bond is reflected across this plane. This method directly illustrates the “mirror image” aspect of enantiomers. Regardless of where the mirror is placed (side, top, bottom, front, or back), all reflections of a chiral molecule will represent the same enantiomer. This is similar to how a digital artist might use a mirror tool to create a symmetrical pattern; the reflected image is distinct yet related to the original.
-
The Wedge and Dash Inversion Method: This is often the most practical approach. Redraw the molecule, but for every chiral center, switch the wedge bonds to dash bonds and the dash bonds to wedge bonds. All other bonds (those in the plane of the paper) remain as they are. This action effectively inverts the configuration of that chiral center (R becomes S, and S becomes R), thereby generating the enantiomer. For example, if a chlorine atom is shown with a wedge bond in one enantiomer, drawing its enantiomer would involve showing that chlorine with a dash bond. This method underscores the precise inversion of spatial arrangement that characterizes enantiomers.
A crucial point, often a source of error, is to not combine both methods simultaneously. If you draw a mirror reflection and also switch all the wedges and dashes, you will inadvertently revert back to the original molecule, simply rotated 180 degrees. This is akin to applying a reflection filter and then an inversion filter in photo editing software – the result might cancel out the intended transformation, leading back to the original visual. Accuracy in molecular representation, like in any visual design, depends on a clear understanding of the transformations being applied. Tophinhanhdep.com, dedicated to visual precision, emphasizes the importance of mastering such techniques for clarity and correctness in depicting complex structures.
Beyond Enantiomers: Introducing Diastereomers
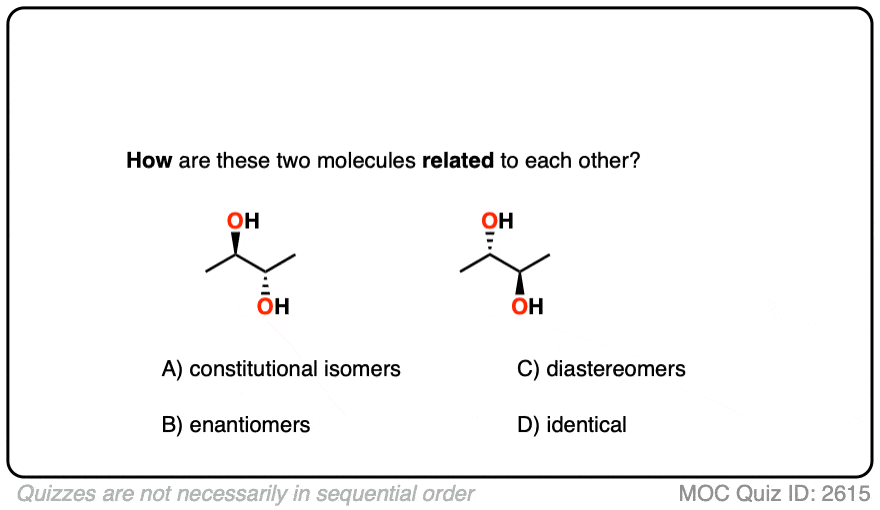
While enantiomers are fascinating due to their mirror-image relationship, they are just one type of stereoisomer. Stereoisomers are molecules that have the same connectivity of atoms but differ in their spatial arrangement. To fully appreciate the unique nature of enantiomers, it’s essential to understand another class of stereoisomers: diastereomers.
Diastereomers are stereoisomers that are not mirror images of each other. This definition, though seemingly broad, precisely captures their relationship. They share the same connectivity, but their 3D orientations are different in a way that does not make them mirror images. For a molecule to have diastereomers, it must possess at least two or more chiral centers.
Here’s how to differentiate:
- Enantiomers: All chiral centers are inverted (e.g., an (R,R) molecule’s enantiomer would be (S,S)).
- Diastereomers: Some, but not all, chiral centers are inverted (e.g., an (R,R) molecule could have diastereomers like (R,S) or (S,R)).
Consider a molecule with two chiral centers, say (R,R). Its enantiomer would be (S,S). However, molecules like (R,S) and (S,R) would be diastereomers of the (R,R) form (and also diastereomers of the (S,S) form). The (R,S) and (S,R) forms themselves would be enantiomers of each other. This complex interplay of configurations highlights the richness of molecular stereochemistry, much like how a theme in aesthetic photography can have multiple variations, each distinct yet related.
It’s also important to note that achiral molecules can be diastereomers of each other. A classic example is cis and trans isomers in alkenes or cycloalkanes. Cis-2-butene and trans-2-butene are stereoisomers because they have the same connectivity but different spatial arrangements around the double bond. They are not mirror images of each other (they are generally achiral themselves), so they are classified as diastereomers. This extends the definition of diastereomers beyond just molecules with chiral centers, encompassing a wider range of spatial differences.
Understanding the distinctions between enantiomers and diastereomers is critical for predicting molecular properties and reactivity. Diastereomers, unlike enantiomers, typically have different physical properties (melting points, boiling points, solubility, etc.) and different chemical reactivities, making their separation much easier than separating enantiomers. This is a fundamental concept in chemical synthesis and drug discovery, where the precise control of molecular spatial arrangement is paramount, much like the precise control of composition and light in creating truly beautiful photography or graphic design.
Conclusion: The Aesthetic of Asymmetry and the Power of Molecular Design
From the vast, awe-inspiring landscapes captured in nature photography to the intricate patterns of abstract art, Tophinhanhdep.com celebrates the myriad forms of visual expression. Our exploration into the world of enantiomers and molecular chirality reveals another profound dimension of visual appreciation: the subtle yet powerful aesthetics inherent in molecular structure itself. The question, “are enantiomers mirror images?”, has led us through a fascinating journey into the principles of non-superimposability, the crucial role of stereogenic centers, the precise language of R and S configurations, and the unique interaction of molecules with light.
We’ve seen that enantiomers are indeed mirror images, but crucially, they are non-superimposable mirror images – a distinction that is visually compelling and functionally significant. This ‘handedness’ at the molecular level dictates everything from the effectiveness of a drug to the specific scent of a fragrance. Just as a high-resolution image reveals minute details that might be missed at a lower resolution, understanding molecular chirality uncovers a hidden dimension of structural elegance and functional precision in the natural world.
The precision required to identify, draw, and understand enantiomers mirrors the meticulous craft behind digital photography and visual design. Whether it’s choosing the right angle for a stock photo, optimizing an image for clarity, or crafting a compelling graphic design, attention to detail and spatial arrangement is key. Similarly, in chemistry, these seemingly small differences in molecular “visual design” can lead to profoundly different behaviors, impacting our health, environment, and technological advancements.
At Tophinhanhdep.com, we believe that an appreciation for visual beauty extends to all scales, from grand vistas to the invisible dance of molecules. By delving into concepts like molecular chirality, we not only expand our scientific understanding but also cultivate a deeper appreciation for the intricate and often elegant “visual design” inherent in nature itself. We invite you to continue exploring the world’s visual wonders, both seen and unseen, and discover how fundamental scientific principles contribute to the broader tapestry of beauty and complexity that inspires our collections of wallpapers, backgrounds, and aesthetic photography.